Reaction engineering: carbon-neutral energy conversion and storage
Current carbon-intensive industries require environmentally-benign energy conversion technologies to satisfy both energy demands and environmental regulations. In addition, carbon-neutral technology that can minimize carbon emission is in the spotlight through achieving a hydrogen society that is recently pursued worldwide. Various alloy catalysts are used in this process, but due to the lack of understanding of complex surface reaction mechanisms, there is a huge gap between catalytic reactions at micro-scale and reactor design at pilot-scale. Our goal is to provide an integrated solution to reactor design at the continuum level of macro-scale based on the investigation of the complex catalytic surface reaction mechanism at micro-scale.
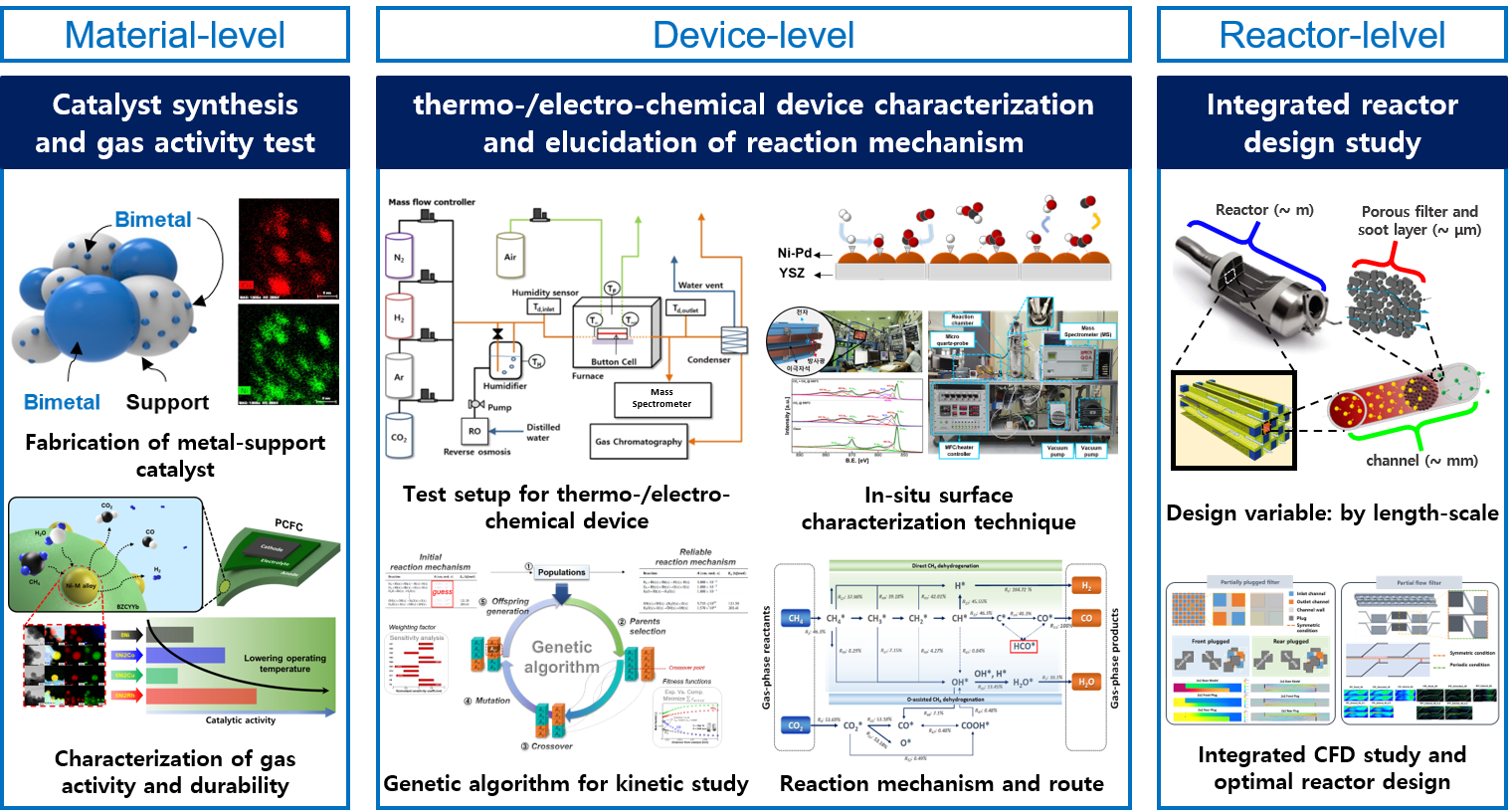
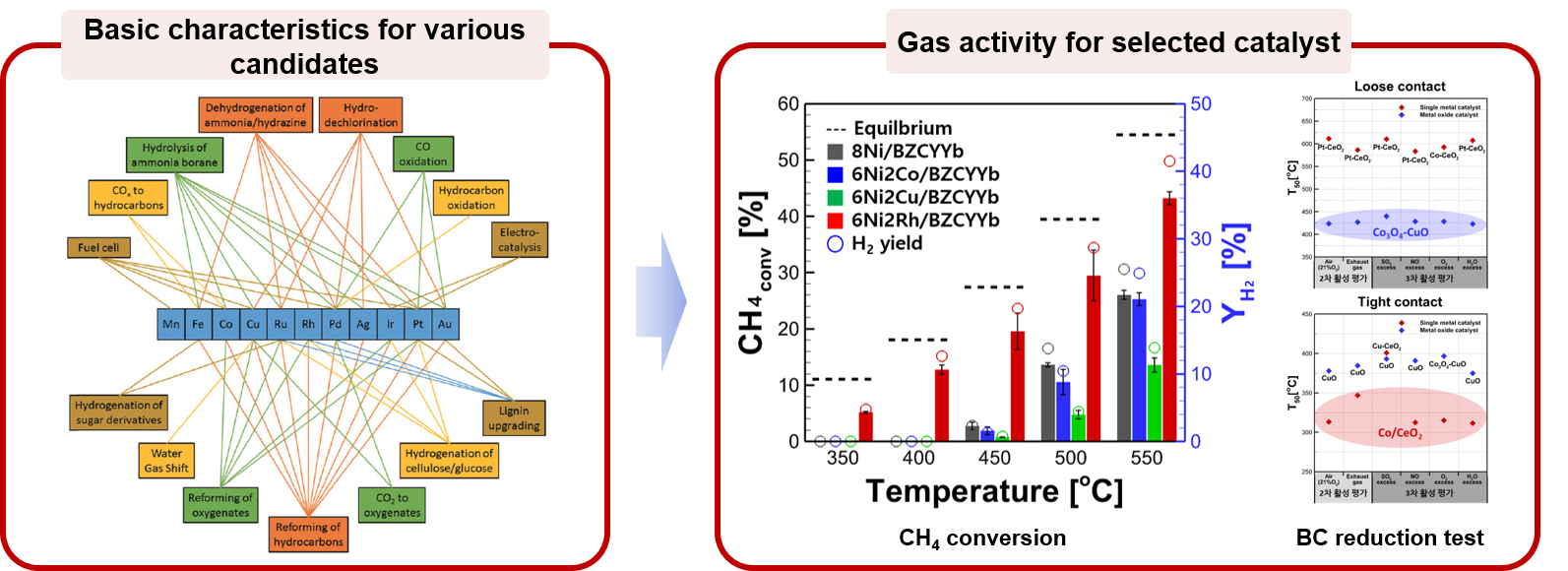
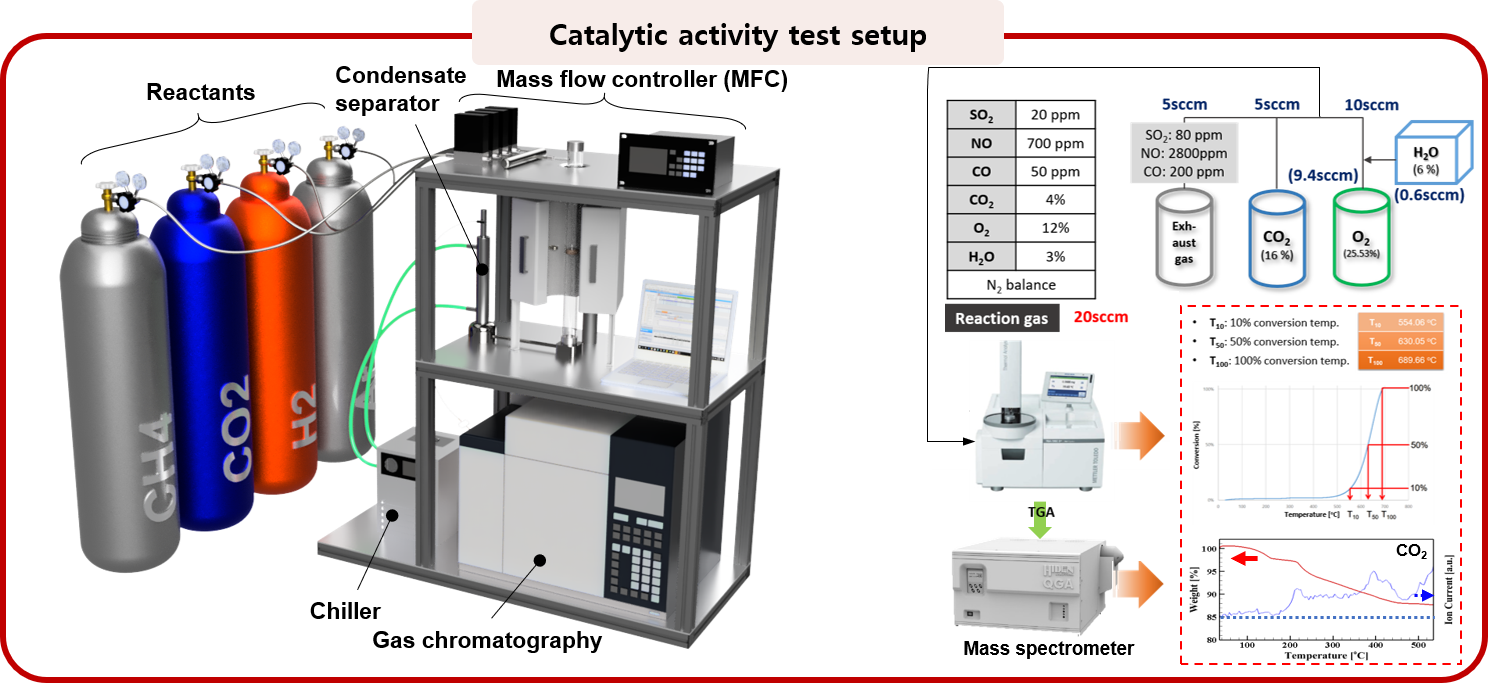
1. Catalyst screening and basic performance test for hydrocarbon fuel reforming and black carbon oxidation
Abundant hydrocarbon (i.e., natural gas) resources and its supply promote extensive research to develop efficient reforming technologies. Natural gas reforming technologies are largely divided into methods using H2O, O2, and CO2, and each method could be applied to a different field due to different yields of the final product. In the MES laboratory, for the purpose of promoting hydrocarbon reforming reaction and securing economic feasibility, a catalyst material with high performance and durability is selected and is applied to a device to improve practical performance. For exhaust gas purification by reduction of black carbon emission, black carbon oxidation catalysts are investigated. Several types of oxide materials are selected for its application at a device level such as a diesel particulate filter. To evaluate oxidative ability and durability of materials, their performance is studied not only under air condition but also under actual exhaust gas composition.
Main topic
: powder screening (SRM/DRM/BC reduction)
References
[1] Hong et al. Journal of Materials Chemistry A, 9 (2021) 6139.
[2] Lee et al. (DRM screening on Ni-M/Al2O3) under review.
[3] Min et al. (Black carbon oxidation catalyst) in preparation.
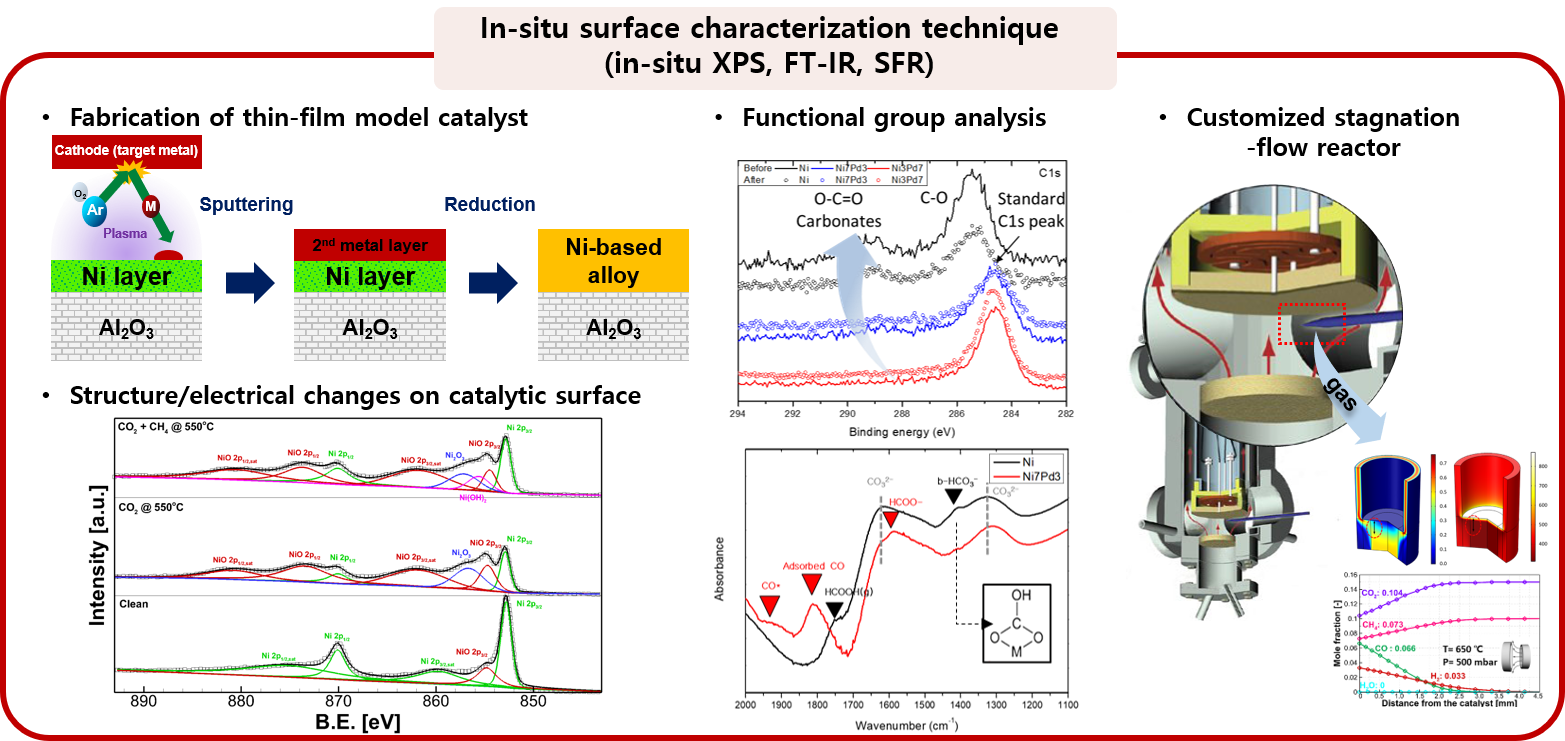
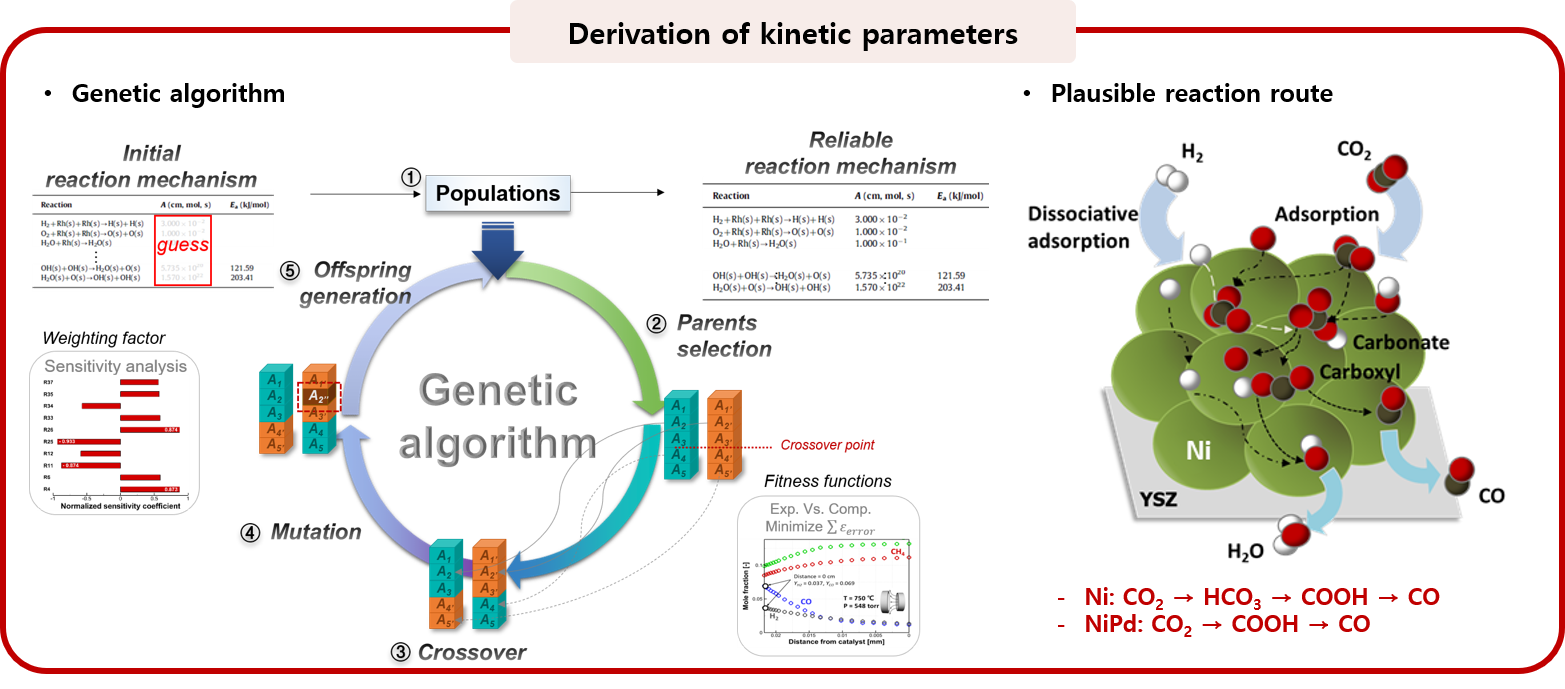
2. Elucidation of heterogeneous reaction mechanism
The transition metal is a type of catalyst material that is widely used in various energy conversion devices due to its low cost and high reactivity, but has a disadvantage of serious degradation (high temperature sintering and carbon coking). Catalytic reaction mechanism on such catalyst has a very complex reaction route, leading to the lack of understanding about detailed reaction kinetics. The fundamental understanding of the reaction mechanism is a key factor that has a great influence from the selection of suitable catalytic materials to integrated heat/mass transport reactor design. To meet these needs the MES laboratory elucidates surface reaction mechanism by using in-situ characterization techniques, and improves products yield and selectivity by overcoming rate-limiting steps.
Main topic
: in-situ characterization techniques (in-situ FT-IR, in-situ XPS, SFR)
: GA-assisted optimization with the 1D reacting-flow model
References
[1] Bae et al. (GA assisted kinetic fitting method) in preparation.
[2] Bae et al. (elucidation of Ni-Pd reaction mechanism on C1 gas reforming) in preparation.
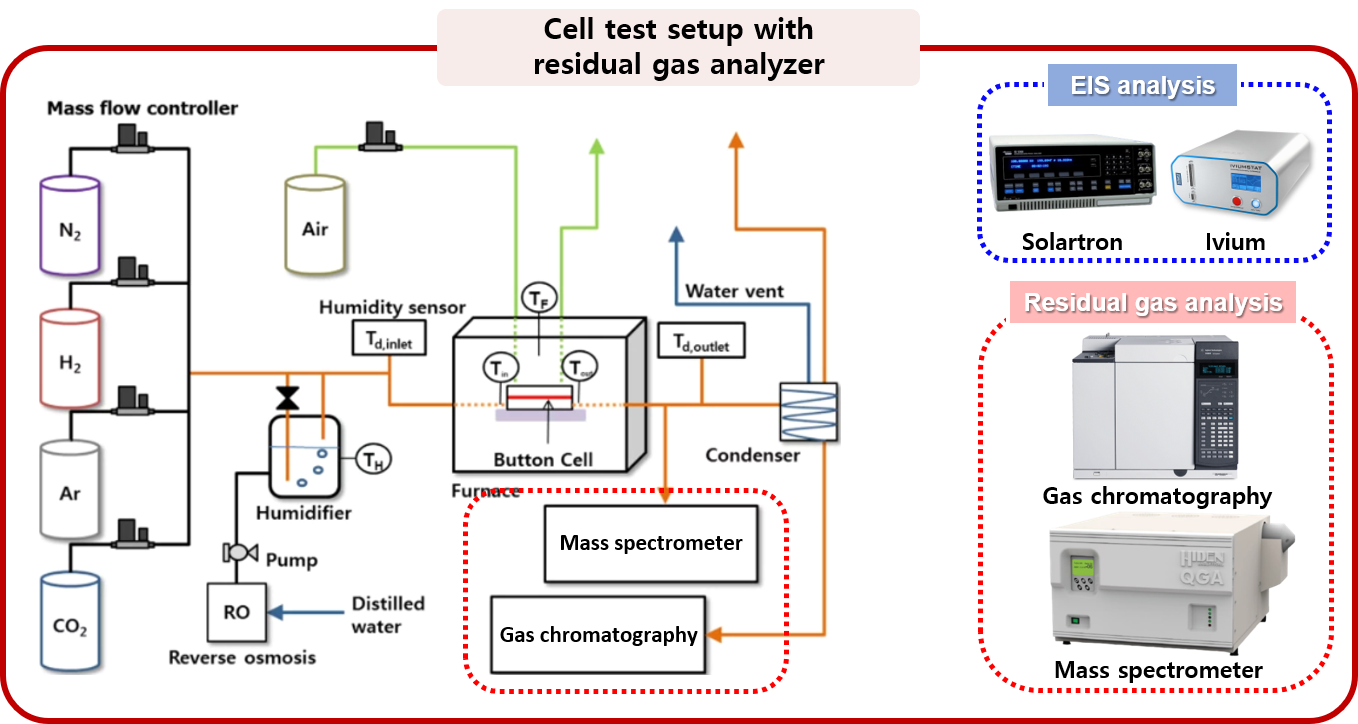
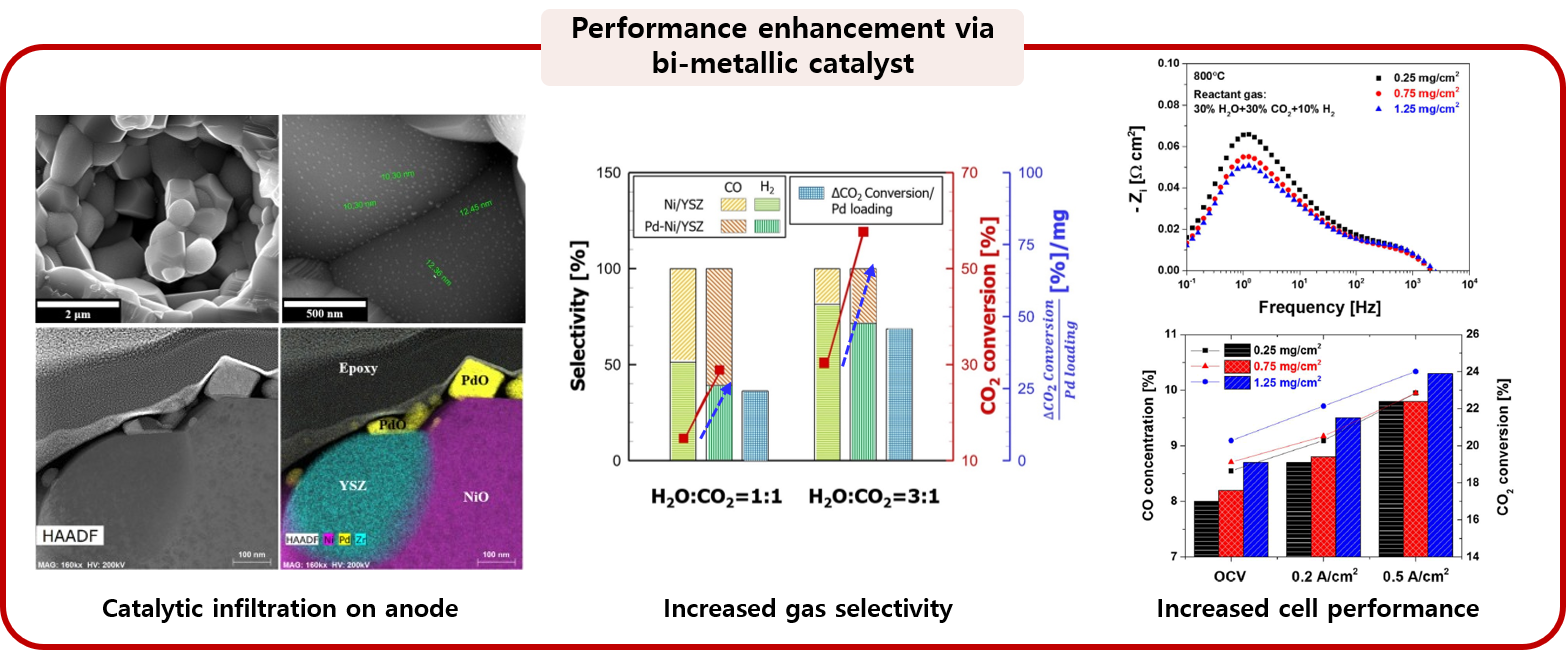
3. Energy conversion and storage devices using electrochemical device
The electrochemical system operates through an oxidation/reduction reaction constructing potential difference between anode and cathode, and there are two modes of operation: a fuel cell mode and an electrolysis mode. Since the operating conditions of the electrochemical system handled in MES are high temperature, the electrochemical reaction occurring at the electrode interface coincides with the thermochemical reaction occurring at the catalyst surface. However, traditional electrochemical analysis techniques such as I-V scanning and EIS analysis have the limit on probing the reaction characteristics, restricting the measurement to the property caused by electron transfer in the both electrodes. Therefore, in the MES laboratory, we intend to provide an integrated analysis for gas reactions occurring inside the device by combining the electrochemical analysis technique and the real-time residual gas analysis system.
Main topic
: H2O/CO2 co-electrolysis with bi-metallic catalysts
: design of an in-situ potentiodynamic gas probing system
: (nano-structured electrode for molecular diagnostic)
References
[1] Kim et al. Journal of the Electrochemical Society, 163 (11) (2016) F3171.
[2] Kim et al. Applied Catalysis B: Environmental, 200 (2017) 265.
[3] Kim et al. Journal of Power Sources, 378 (2018) 369.
[4] Jeong et al. International Journal of Energy Research, 43(9) (2019) 4949.
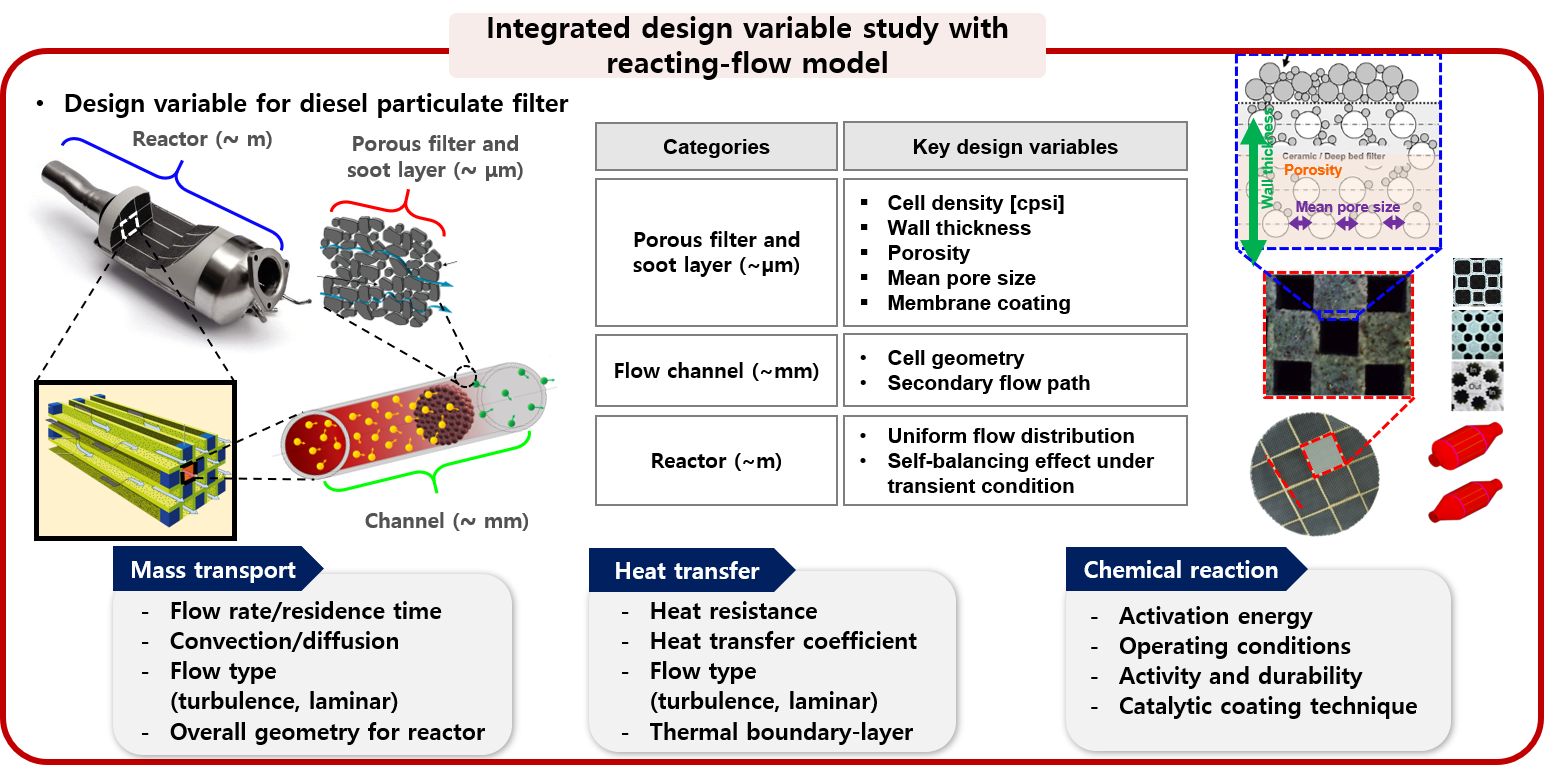
4. Optimized reactor design by multiphysics analysis
Field of heterogeneous catalysis is highly interdisciplinary, requiring the cooperation between chemists and physicists; between surface scientists and reaction engineers; between theorists and experimentalists; between spectroscopists and kineticists; between material scientists involved with catalysts synthesis and characterization. Due to this complexity and difficulty, there is a lack of integrative reactor design study from micro-kinetic reaction to continuum transport phenomena. Therefore, the MES laboratory proceeds with an optimal reactor design based on the in-house elucidated fundamental mechanism through analysis of the local thermodynamic state occurring on the catalyst surface.
Main topic
: aerodynamic filtrated BC reduction reactor with oxidation catalysts
References
[1] Bae et al. Energy Conversion and Management, 165 (2018) 405.
[2] Bae et al. Energy Conversion and Management, 201 (2019) 112152.